Slippery substance
Water continues to reveal mysteries and surprises as researchers investigate its structure.
by Heather Rock Woods
|
|||||||||||||||
Liquid water is essential, beautiful, powerful, and completely strange.
The substance whose properties we take for granted actually acts like no other liquid on the planet. It expands when it freezes, holds heat exceptionally well (allowing Europe to have a moderate climate instead of a Siberian one), and has a high surface tension (allowing plants to pull water up from their roots). Fish and other lake life survive the winter because water's maximum density is several degrees above the freezing point; cold water sinks to the bottom, but water close to freezing resides at the top, ultimately forming a protective layer of ice. Water can put out fires, even though its individual components–hydrogen and oxygen–incite flames. And liquid water is an ideal solvent for the biological molecules and systems that help us circulate oxygen in our bodies, reproduce, and exist.
"Many of these unique properties are the basis of our existence," says Anders Nilsson, a chemical physicist at the Stanford Synchrotron Radiation Laboratory (SSRL), part of Stanford Linear Accelerator Center.
Everyone knows the chemical formula for water, but as strange as it sounds, scientists have only recently begun to discover what liquid H2O really looks like and how it really behaves. Using new methods at synchrotron radiation laboratories, researchers have come to some startling, and sometimes controversial, conclusions.
"The impact of understanding the structure of water correctly is important to every part of biology and many parts of chemistry. If we don't even understand pure, simple liquid water, how are you going to understand how life works," says Uwe Bergmann, an SSRL scientist who collaborates with Nilsson.
Scientists have found creatures that don't need oxygen, but so far liquid water appears indispensable to life. Even exobiologists searching for life on other planets look for signs of liquid water.
Liquid water is a slippery substance to pin down in the lab. Normal imaging techniques work for ice, which has a crystalline structure, and for the far-apart molecules of water vapor. But liquid water, in bulk, is disorganized and always changing–hooking up with and breaking up with near neighbors a trillion times a second. The normal synchrotron x-ray technique requires the water sample to be in vacuum, which causes the sample to quickly evaporate.
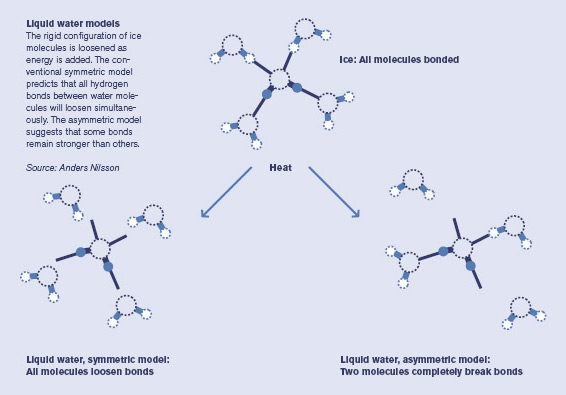
Over decades, theoretical calculations and some experimental data helped to build up a picture of how liquid water works. Solid ice is clearly known: a water molecule bonds to four other ones in a tetrahedral shape. When you add heat to melt the ice, the prevailing theory holds that each water molecule loosens its bonds a little, but still essentially maintains all four bonds.
But when Nilsson did the experiment, the spectrum of liquid water he saw reminded him of the surface of ice–where water molecules abruptly run into air (or vacuum in a lab), and thus have only two bonds. After two to three years of careful research and analysis, aware that they were up against 50 years of accepted thinking, the collaboration published their stunning results, claiming that ice melts asymmetrically, with each H2O holding strong to two other water molecules, but letting the other two go. (See diagram below.)
"Our results didn't fit the established wisdom of water," Nilsson says. "It was hard to get published, but then it became one of the top ten results of 2004 in Science." Since then, the "asymmetric concept" of liquid water bonding has attracted significant research interest.
The making of an experiment
Many structure of water studies rely on synchrotron techniques because synchrotrons provide powerful, tunable x-ray beams that can be set to different x-ray energies to see how much of the specific energies are absorbed or transmitted by the materials being studied. The results are usually displayed as a spectrum (see figure below). Researchers are also applying neutron diffraction, infrared absorption, and other techniques of varying sensitivities to different aspects of the problem.
Nilsson was an expert in developing synchrotron x-ray spectroscopy techniques to understand chemical bonds on surfaces. He happened into water research when he decided to apply x-ray spectroscopy techniques to liquids, starting with an amino acid. So that they could take into account the "background" water, his group ran a spectrum on the water solution.
"I assumed at that point that liquid water was not interesting, that it was well understood. I have a library in my mind of all spectra, and when we measured the liquid water spectrum the first time I realized it looked very different than ice. It looked more like the surface of ice–I then understood immediately we had something interesting," says Nilsson.
The group did the initial experiments at the Advanced Light Source at Lawrence Berkeley National Laboratory and at the Advanced Photon Source at Argonne National Laboratory, while the SPEAR synchrotron at SLAC was being upgraded. The collaboration included experimentalists and theorists from SSRL, Stockholm University and Linköping University in Sweden, BESSY in Germany, and Utrecht University in the Netherlands. The team overcame the formidable challenges of keeping water liquid rather than vapor in the vacuum conditions necessary for soft-x-ray experiments.
Soft x-rays have the right energy to probe the electrons around light elements like oxygen. Hydrogen atoms are nearly invisible to x-rays because they contain just one electron.
X-ray absorption spectroscopy (XAS) reveals where electrons are congregating and where they are absent, information that tells researchers about bonds because an atom's outer electrons are the ones that form bonds with other atoms.
When x-rays hit atoms, electrons can jump from their positions in the innermost shells near the nucleus to higher-energy shells–like bumping Mercury out to Venus's orbit–but only if the energy of the x-ray precisely matches the energy required to make the jump. Also, electrons can only go to shells with room in them; if the closest shell is full, it requires a step up in energy to bump the electron out of its inner shell.
"By looking at the energy required to knock out an [inner shell] oxygen electron, you will learn about how the H2O molecule is bonded," says Bergmann.
For an oxygen atom, like the main atom in a water molecule, researchers first tune the x-ray beam to 535 electronvolts, the exact amount of energy it takes to eject an inner oxygen electron to the first unoccupied shell.
Then by applying a range of energies to the water sample, researchers create an energy spectrum with peaks that show which shells have room for electrons. If both hydrogen atoms in a water molecule are attached to other H2O molecules, the oxygen atom will share its electrons evenly within the water molecule, and there will be a characteristic peak in the spectrum. But if only one of the two hydrogen atoms has formed an external bond, there will be two electronic states–two different local environments for the electrons–that show up in the spectrum as two sets of peaks (see figure in deconstruction section).
The role of theory
Theory plays more than just a predictive role in this research. The nature of the experimental techniques used requires simulations and calculations to interpret the raw spectra and diffraction patterns obtained.
In the case of Nilsson's group, his team worked very closely with the theory group at Stockholm University.
"The only thing we have determined experimentally is that the internal oxygen-hydrogen bonds are different from each other," says Nilsson.
Lars Pettersson of Stockholm University used molecular dynamics simulations, which model the motions of many individual molecules, to compute hundreds of spectra. Only when the simulations had one hydrogen externally bonded and the other unbonded did the computer- generated spectra match the experimentally gathered ones.
"We looked at many different possible structures. In the beginning it was trial and error, then you can become more systematical," he says.
Pettersson says there is much discussion about the accuracy of the simulations, but points out the calculations have been calibrated on well-known compounds, and the team has 15 to 20 years of theoretical and experimental experience.
The asymmetric model is a boldly different way to understand liquid water and it might help explain some of water's anomalies, like becoming more mobile rather than more viscous under pressure.
"What we need to do is establish whether the asymmetric concept works or whether it doesn't work," says Alan Soper of Rutherford Appleton Laboratory in the United Kingdom. "As it turns out, it is quite difficult to find some experimental measure that will categorically deny the asymmetric concept."
Soper studies the structure of water using neutron beams at RAL's ISIS facility. Neutron diffraction gives precise information about the distances between atoms from probing the nuclei of atoms, rather than the electrons. He can study regular water and heavy water (in which the hydrogen atoms have an added neutron that doubles their mass).
He has reviewed much of the data to date (although not XAS data), and found that they can be equally well described by both models, the symmetric and asymmetric.
"I've tried to maintain a sitting on the fence attitude," says Soper. "[The asymmetric model] has not been fully proven yet. I want to give room for these ideas to flourish or be denied."
The discussion and debate will continue this June in Stockholm at a roundtable that Nilsson and Pettersson are organizing on the structure of water. Especially since the 2004 paper came out, the research on water has been very active, with many groups from around the world, including members of the original collaboration, pursuing the structure of water using various techniques and experimental conditions.
"Our spectrum by itself is not going to resolve this issue, we need many measurements," says Bergmann.
The original claim rests not just on XAS data, but on x-ray Raman scattering, a relatively new hard (higher-energy) x-ray technique put to efficient use by Bergmann. The technique can be used on high-temperature and high-pressure water samples because no vacuum is required. The Raman spectra for water at different temperatures are part of the raw experimental data that indicate a large structural difference between ice and liquid water. The spectrum for water at 1°C looks dramatically different from ice, yet very similar to 99°C water. If a water molecule's four external bonds all gradually weakened, one would expect a gradual or continual change in the spectrum over those temperatures.
|
Still mysterious
Some of the current experiments are looking for the larger-scale structure of bulk water and the significance of hydrogen bonds, which are probably the secret to water's numerous anomalies. Water is unique in that it can form from one to four hydrogen bonds (one with each hydrogen atom and two with the oxygen atom). The bonds are weaker than typical covalent bonds, allowing biological molecules to form and break bonds easily. This is how DNA can be unzipped to make copies and then be zipped together again, and how proteins fold and participate in vital chemical reactions.
"Hydrogen bonds are how nature comes alive, how nature makes changes," says Nilsson.
Because hydrogen bonds like to connect things in a straight line, water molecules with only two strong hydrogen bonds will possibly form flat chains or rings. The flat sheets could stack in layers, loosely held together by the other two very weak hydrogen bonds.
Scientists, of course, want to build a picture not just of liquid water but of all its phases, understanding at what temperatures and pressures it's a gas, a liquid, a solid, or some strange hybrid. The behavior of water at unusual temperatures and pressures will help scientists understand exotic environments, like those on another planet or a comet, or in the hot, high-pressure conditions around undersea volcanoes where strange life forms exist.
"The phase diagram of water is extremely rich," Bergmann says.
At temperatures about 400°C and pressures of 300 atmospheres, water exists in a "supercritical" state where it's neither vapor nor liquid, but appears to contain clusters of liquid within a gas. Supercritical water is, for example, used in environmental cleanup.
|
Researchers expect that new experimental facilities will improve the picture of water. A proposed beamline for the SPEAR3 synchrotron at SSRL would focus on Raman scattering experiments for water and other materials. SLAC is currently building a hard x-ray free-electron laser, the Linear Coherent Light Source (LCLS), which will make motion pictures of atoms and molecules. Bergmann and Nilsson have already proposed experiments to study structural changes in water in real time.
"The important thing is to get this right," says Pettersson. "Water is very important."
What is currently known about water is a mere drop in the bucket, but researchers continue to reveal the mysteries of this most everyday substance.
Click here to download the pdf version of this article.














